SRFP011: Association Between Electronic Nicotine Delivery Systems Use and Breastfeeding Duration (Pearls)
Megan McBride; Zelalem Haile, PhD, MPH
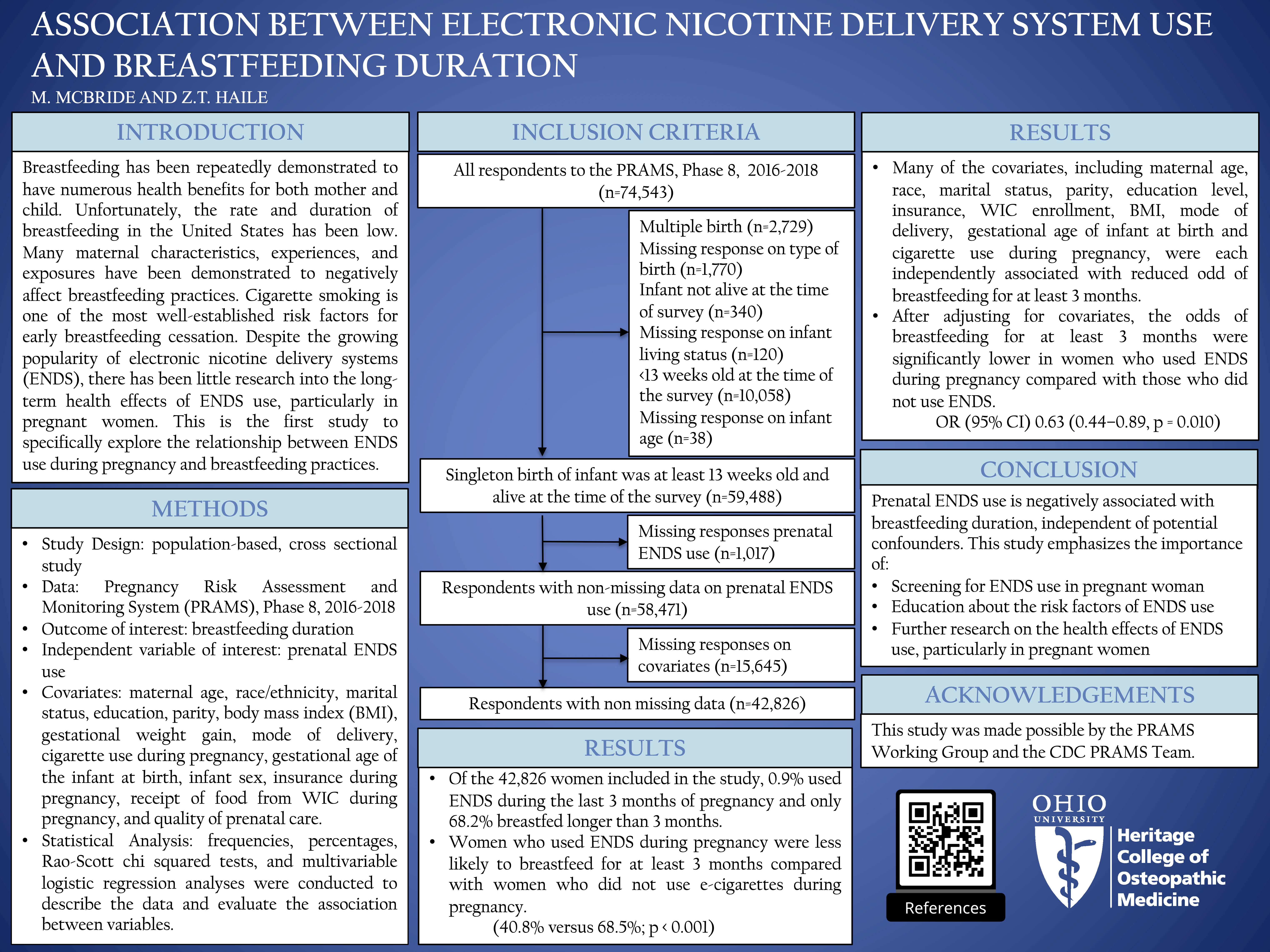
Abstract
breastfed for the recommended duration of time. Previous studies have identified several demographic, socioeconomic, biological, and behavioral factors that impact breastfeeding practices. Studies examining the
influence of electronic nicotine delivery systems (ENDS) on breastfeeding practices are currently lacking.
Materials and Methods: This population-based, cross-sectional study used data from the 2016–2018 Pregnancy
Risk Assessment and Monitoring System (n = 42,827). Chi-squared tests and multivariable logistic regression
analyses were performed.
Results: The prevalence of prenatal ENDS use was 0.9%. Only 40.8% of women who used ENDS during
pregnancy breastfed for at least 3 months compared with 68.5% of women who did not use ENDS during
pregnancy. In the multivariable model, the odds of breastfeeding for at least 3 months were significantly lower
in women who used ENDS during pregnancy compared with those who did not use ENDS; odds ratio (95%
confidence interval) 0.63 (0.44–0.89; p = 0.010).
Conclusion: Prenatal exposure to ENDS is negatively associated with breastfeeding duration, independent of
potential confounders. This finding suggests that screening for ENDS use during pregnancy can play a vital role
in identifying women at-risk for suboptimal breastfeeding and offering ongoing support to improve breastfeeding practices.
Keywords: electronic nicotine delivery systems, breastfeeding duration, prenatal period, PRAMS
Dennis Baumgardner, MD
11/19/2021Interesting study - thank you!