PRP046: Human Papilloma Virus self-sampling option to increase cervical cancer screening rates
Christina Scartozzi, DO; Jennifer Moss, PhD; Kelsey Stoltzfus, MPH; Brittney Calatayud, BS
Abstract
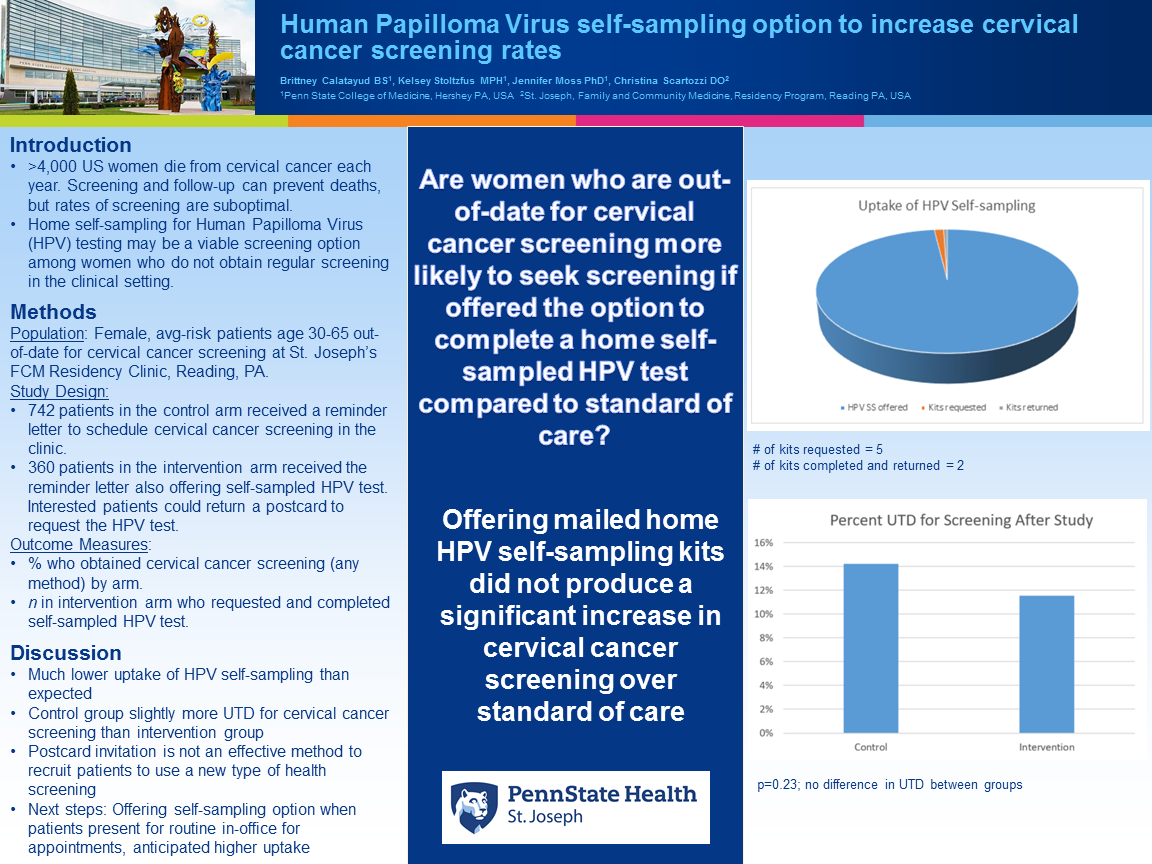
Context: The American Cancer Society estimates 4,290 women in the United States will die from cervical cancer in 2021. Screening and appropriate follow-up can prevent deaths. Rates of screening are suboptimal and vulnerable subgroups (including women in rural areas, racial/ethnic minorities, women with low socioeconomic status, and women who are underinsured) are even less likely to be screened. Home self-sampling for Human Papilloma Virus (HPV) testing may be a viable screening option among women who do not obtain regular screening in the clinical setting. This study uses real-world procedures that could pragmatically be adopted in primary care clinics, i.e., evaluating a reminder letter with a post card option to request an HPV self-sampling kit. Objective: To evaluate the effectiveness of offering self-sampling for high-risk HPV testing to increase cervical cancer screening rates. Study Design: Pragmatic feasibility study Setting or Dataset: Primary care clinic in an underserved, mainly Latinx community Population studied: Inclusion criteria: Female patients age 30-65 out-of-date for cervical cancer screening and are not at greater than average risk for cervical cancer. Exclusion criteria: Being pregnant, incarcerated, having a greater than average risk for cervical cancer. Using a 2:1 randomization scheme, we will randomize 807 participants to the control arm and 404 participants to the intervention arm. Intervention/Instrument (for interventional studies): Women in the control arm will receive a letter notifying them that they need to be screened and they should contact their primary care provider, according to standard clinical procedures. Women in the experimental arm will receive a slightly modified version of this letter informing them of a self-sampling option and a pre-addressed post card to return if interested in self-sampling. Outcome Measures: Proportion of participants who obtain cervical cancer screening (in clinic or with self-collected samples for HPV testing) by arm within three months of the initial mailing. We will use chi-squared tests to compare these proportions, using an alpha of .05 and a beta of 0.80. Results: We assume a 15% response rate from the control group and a 30% response rate for the intervention group for a final n=122 for each group. Conclusions: The proportion of participants who obtain cervical cancer screening by arm will be reported.
Diane Harper
harperdi@med.umich.edu 11/21/2021Congratulations Jennifer! We must continue to spread the word that home based colorectal and cervical cancer are the best possible ways to screen the average risked person for respective cancers! I look forward to the future work you will be doing! Thank you for sharing with us at NAPCRG.