PRP032: Essential content for reports of primary care research? Delphi study of the international primary care research community
Pallavi Prathivadi, MBBS, MMed (Pain Mgt), BMedSc (Hons), DCH, FRACGP; William Phillips, MD, MPH, FAAFP; Liz Sturgiss, MD, PhD, BMed, MPH, FRACGP
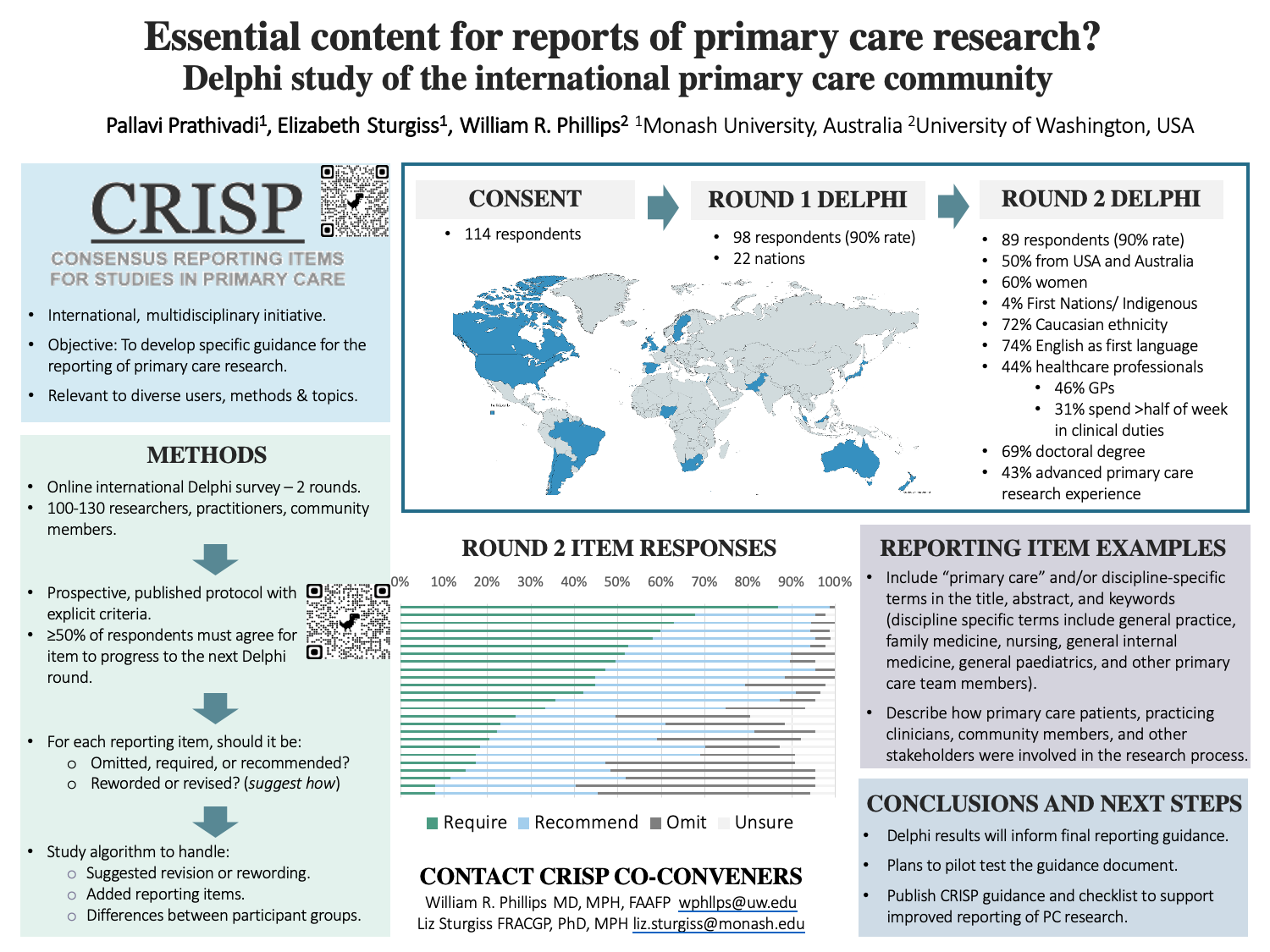
Context: Current guidelines for reporting health research do not adequately address the complexities of the patients, problems, clinicians, and settings that are characteristic of primary care (PC). Consensus Reporting Items for Studies in Primary Care (CRISP) is an international, interdisciplinary initiative to support the PC research community and enhance the quality of research reporting. Objective: To develop specific guidance for the reporting of PC research. Study design: Online Delphi survey. Setting: International PC community. Population studied: 100-130 diverse members of the international PC community with varied perspectives and expertise, including patients, clinicians, researchers, readers, reviewers, and editors. Intervention: The prospectively published research protocol outlined a modified Delphi procedure to identify and prioritize essential items to be included in reports of PC research. Potential reporting items were informed by two previously published CRISP surveys of researchers and practicing clinicians. The Delphi panel was recruited to reflect an international, interdisciplinary PC research community from volunteers from prior CRISP surveys and CRISP working group networks. Delphi participants considered statements of reporting items in up to three sequential rounds of confidential online questionnaires completed using Qualtrics software. Participants were asked: 1) if the item should be considered essential or encouraged in PC research reports, 2) to give their reason, and 3) offer any suggestions for improved wording. Subsequent survey rounds provided participants with a summary of the responses of all participants and their individual responses to the previous round. Using participant comments and suggestions, the Working Group edited statements for the next round. Consensus was be determined for each statement by the percentage of participants in agreement (at least 50%). Outcome measures: Consensus agreement after 2 or 3 Delphi rounds on statements to be included in PC research reports. Outcomes to be reported: The final Delphi results and Working Group editing will identify the final draft list of statements to be included in the CRISP guidance on PC research reporting.
Tim olde Hartman
tim.oldehartman@radboudumc.nl 11/19/2021Great research and great to be part of the CRISP initiative. We're going to hear from CRISP many times in the near future.