PRP073: Reflux in infants: a quality improvement assessment leading to a research project: A protocol for a pilot study
Isabel Rodrigues, MD, MPH, MPH; Yessica Ngalula; Jonathan Lefebvre, MD, CCFP; Annie-pier Crevier, IBCLC, inf clin; Katherine Perey, MD; Valérie Limoges; Marie-Eve Lavoie, PhD, RD; Xin Yu Yang
Abstract
Context Gastroesophageal reflux (GER) is a normal physiological process that occurs frequently in infants. Various guidelines published over the past 10 years prioritize a comprehensive approach based on parental education and non-pharmacological solutions in the initial management of GER. During a quality improvement study in two academic settings in Quebec, Canada, a sub-optimal practice, with regard to recent guidelines, revealed that treatment based solely on a non-pharmacological approach was initiated in only 52% of children. Also, among the children who received medication for the treatment of GERD, 65% of the prescriptions were done on the first visit.
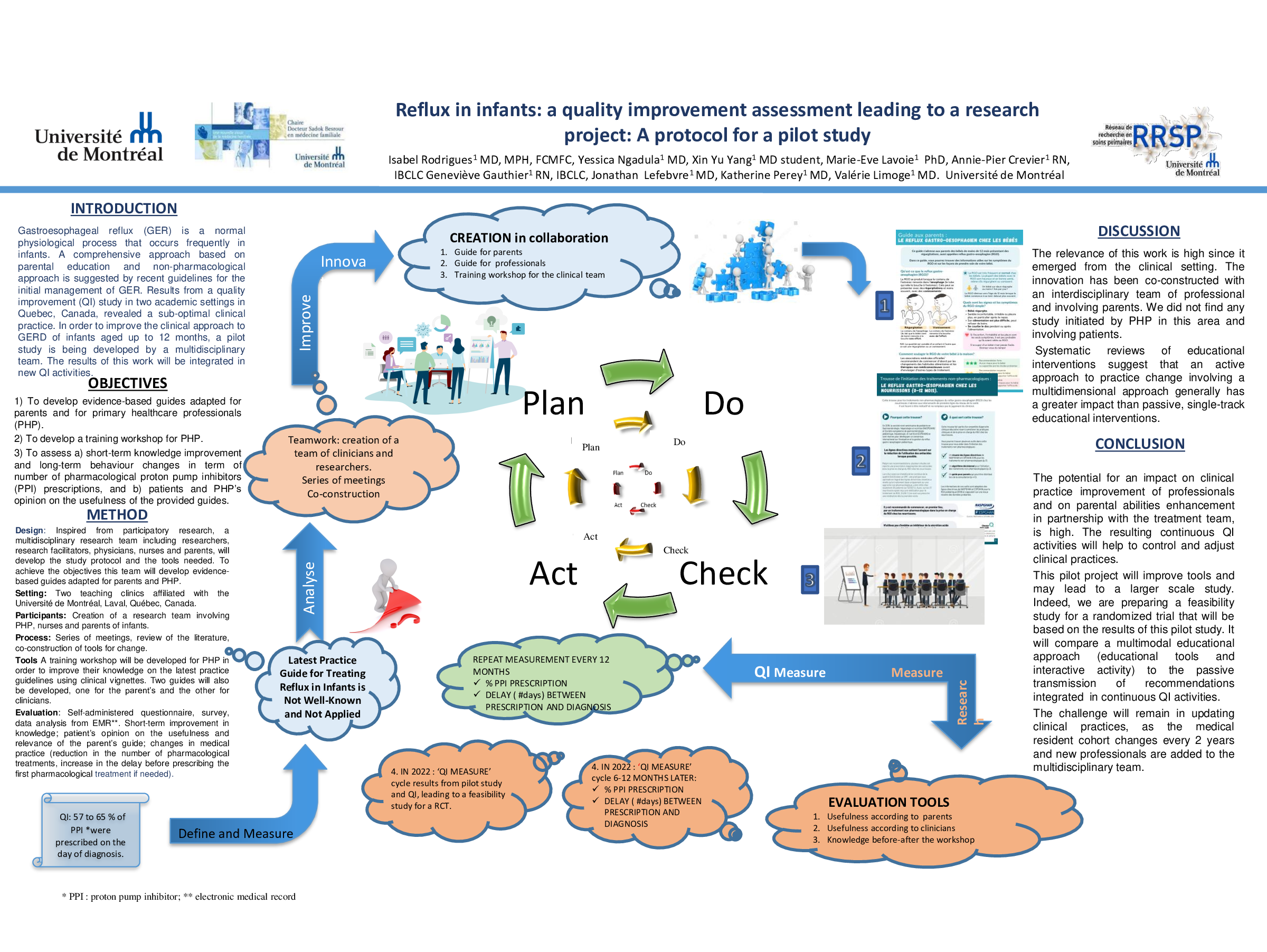
Objectives 1) Develop evidence-based guides adapted for parents and for primary healthcare professionals (PHP). 2) Develop a training workshop for PHP. 3) Assess a) short-term improvement in knowledge and long-term behaviour changes in term of number of pharmacological treatments b) patients and PHC’s opinion on the usefulness of the guides provided.
Method: Inspired from participatory research, a multidisciplinary research team including researchers, research facilitators, physicians, nurses and parents, will develop the study protocol and the tools needed. To achieve the objectives this team will develop evidence-based guides adapted for parents and PHP. A training workshop will be developed for PHC in order to improve their knowledge on the latest practice guidelines using clinical vignettes. Mixed methods process evaluation (self-administered questionnaire, survey, data analysis from EMR) will be developed for: short-term improvement in knowledge; patient’s opinion on the usefulness and relevance of the parent’s guide; changes in medical practice (reduction in the number of pharmacological treatments, increase in the delay before prescribing the first pharmacological treatment).
Conclusion Key indicators will be suggested to guide the development of a Quality Improvement (QI) cycle. The potential for impact, on improving the clinical practice of PHP and on enhancing parenting skills in partnership with the treatment team, is high. The intervention developed in this study will be tested in a randomized trial. The resulting QI cycle emerging from this research project will allow practices to be monitored and adjusted.
Objectives 1) Develop evidence-based guides adapted for parents and for primary healthcare professionals (PHP). 2) Develop a training workshop for PHP. 3) Assess a) short-term improvement in knowledge and long-term behaviour changes in term of number of pharmacological treatments b) patients and PHC’s opinion on the usefulness of the guides provided.
Method: Inspired from participatory research, a multidisciplinary research team including researchers, research facilitators, physicians, nurses and parents, will develop the study protocol and the tools needed. To achieve the objectives this team will develop evidence-based guides adapted for parents and PHP. A training workshop will be developed for PHC in order to improve their knowledge on the latest practice guidelines using clinical vignettes. Mixed methods process evaluation (self-administered questionnaire, survey, data analysis from EMR) will be developed for: short-term improvement in knowledge; patient’s opinion on the usefulness and relevance of the parent’s guide; changes in medical practice (reduction in the number of pharmacological treatments, increase in the delay before prescribing the first pharmacological treatment).
Conclusion Key indicators will be suggested to guide the development of a Quality Improvement (QI) cycle. The potential for impact, on improving the clinical practice of PHP and on enhancing parenting skills in partnership with the treatment team, is high. The intervention developed in this study will be tested in a randomized trial. The resulting QI cycle emerging from this research project will allow practices to be monitored and adjusted.
Diane Harper
harperdi@med.umich.edu 11/21/2021Nice example of the process. Thank you for sharing with us at NAPCRG.